Research Directions:

|
|
|
Surfaces
and interfaces define the boundaries of thermodynamic phases and often
exhibit properties that are strikingly different from those of the
bulk. Most of the surface
science studies focus on studies of solids, but surfaces of even most basic
liquids have not been thoroughly investigated.
What is the nature of interface between liquid and vapor in simplest monatomic liquids? The answer depends on nature of atomic interactions in the two phases.
|
|
|
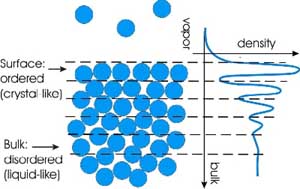
While atomic and
small-molecule liquids are generally unstructured (randomly packed) in the bulk, we have
discovered that at the surface atoms of metallic liquids are stratified into series of
quasi-crystalline layers - so-called "surface layering" phenomena, which
has been a topic of theoretical and computer simulation studies for
several decades but has not been observed experimentally until recently.
All of the metallic liquids studied thus far exhibit surface layering,
while surfaces of non-metallic liquids such as water are not layered.
Recently we have established that the layering is caused primarily by
electronic properties of the metallic liquids through interactions of
quantum Fermi electron gas and classical ionic liquid, rather than
thermodynamic parameters such as surface tension.
[top]
| |
|
|
Unlike
solid surfaces, free liquid surfaces are not static and
are prone to thermally excited surface fluctuations, or capillary waves.
Even in absence of external vibrations, thermally excited fluctuations can significantly complicate direct determination of
atomic structure at the surface. We have developed theoretical and
computational models that allow us to separate the intrinsic atomic structure from roughening caused by surface dynamics.
[top]
|
|
Segregation,
Wetting in Binary Mixtures: A more subtle question is the surface structure of binary fluids, such as mixture of two monatomic liquids. The formation of surface phase strongly depends on the interactions between two types of atoms. Near-surface region composition is defined primarily by the competition of surface energetics and bulk thermodynamics of the two elemental phases. |
|
|
We
have recently demonstrated that in miscible systems (such as eutectic BiSn
alloy) effects of Gibbs adsorption, previously thought to be limited only to surface
monolayer extends further into the bulk - to at least 3
near-surface atomic layers. | |
|
Recently we discovered an unusual surface freezing phenomenon in eutectic AuSi alloy - a formation of well-defined 2D crystalline surface phase with long-range in-plane ordering, as well as anomalously enhanced surface-normal "layering". While in-plane surface ordering has been previously seen in quasi-2D confined systems (Langmuir and Gibbs monolayers), or for long-chain molecular liquids (alkanes and liquid crystals) where anisotropy of the molecules controls ordering, this is the first observation of surface freezing in simple homogeneous system. [top] |
|
|
|
Despite a great number of theoretical research describing physics of solid-melt interfaces, very few existing experemintal techniques can be applied to studies these "deeply buried" interfaces. High-energy x-ray scattering tools can solve this problem and provide unique look at structure, dynamics and reactions at liquid-solid buried interfaces. One of the interesting questions is existence of hard-wall induced ordering in liquid phase, similar to surface layering in metallic liquids. [top] |
|
Reactions
at surfaces and interfaces: Most chemical reactions such as oxidation originate at surfaces and proceed into the bulk. While at solid surfaces the nucleation and growth of oxides begins primarily at defects and impurities, liquid surfaces are defect-free and can be made atomically clean, therefore providing a perfect system to study intrinsic surface oxidation that occurs in complete absence of pre-existing nucleation centers. Our recent studies of oxidation of liquid metals have demonstrated existence of oxidation exposure threshold, which provides a unique view of kinetics of nucleation and growth. |
|
|
Oxidation of defect-free surfaces often results surface crystalline phases previously not observed in the bulk form. [top] | |